in order to synthesize n-phenyl acetamide what should you combine aniline with?
i. Introduction
Tuberculosis, or TB, is a common, and in many cases lethal, communicable diseases acquired by various strains of mycobacteria, ordinarily Mycobacterium tuberculosis, which is the greatest single infection cause of higher bloodshed worldwide [ane]. The World Health Organization (WHO) estimates that 8 meg people become TB every year and three million people will dice yearly from TB if control is non further strengthened. The worsening situation has prompted the WHO to declare tuberculosis a global public health crunch [2,iii]. The emergence of multidrug resistant (MDR) strains of Mycobacterium tuberculosis and the combined epidemics of HIV and tuberculosis, have worsened the situation. On the other hand, tuberculosis incidence rates correlate with poor socio-economic weather. Consequently at that place is an urgent need to develop novel affordable antitubercular drugs with high potency that can provide treatment options for all forms of TB [four,5].
Compounds with a 2-phenoxy-N-phenylacetamide core structure take attracted considerable research interest as these entities have demonstrated a multifariousness of biological activities such equally anti-parasitic [half dozen], anticancer [7] and antiviral furnishings [8]. Recently, in a programme of high throughput screening for inhibitors of M. tuberculosis H37Rv, the hit compound methyl 2-(4-(2-(2,4-dimethylphenoxy)acetamido)phenoxy)acetate (I, Figure 1) [9], was identified to possess stiff antitubercular activity, which indicates that ii-phenoxy-Due north-phenylacetamide may exist a promising scaffold for develop novel antitubercular agents. The synthetic simplicity of the two-phenoxy-Due north-phenylacetamide scaffold provides a strong motivation for the development of constructive and affordable antitubercular agents. To date in that location have been no reports describing the synthesis and antitubercular cess of its derivatives. Moreover, the antibacterial activeness of halonitroanilides were documented and lots of compounds with nitro groups, such every bit nitrofurans and nitroimidazoles, have demonstrated broad spectrum antibacterial activities [10,11]. Encouraged by the prominent activity of the ii-phenoxy-N-phenylacetamide skeleton and the halonitroanilides moiety, we proposed to synthesize some novel compounds containing a 2-(iii-fluoro-iv-nitrophenoxy)-N-phenylacetamide nucleus by a molecular hybridization approach, aiming to search for potential candidates with better antitubercular activities and good safety profiles. Thus sixteen 2-(3-fluoro-iv-nitrophenoxy)-N-phenylacetamide derivatives (II, Figure ane) were designed, synthesized and tested for their in vitro antitubercular action against M. tuberculosis H37Rv by a microdilution method. The most potent one, compound 3m, was assayed for its activities against two resistant Thou. tuberculosis strains isolated from clinical cases. The cytotoxic activities of all the final molecules against half dozen tumor cell lines and normal vero cell line were assayed by MTT analysis.
Figure 1. The chemical structures of methyl 2-(four-(ii-(2,4-dimethylphenoxy)acetamido)phenoxy)acetate (I) and two-(3-fluoro-4-nitrophenoxy)-N-phenylacetamide derivatives (II).
Figure 1. The chemical structures of methyl two-(4-(ii-(2,4-dimethylphenoxy)acetamido)phenoxy)acetate (I) and 2-(3-fluoro-4-nitrophenoxy)-N-phenylacetamide derivatives (II).
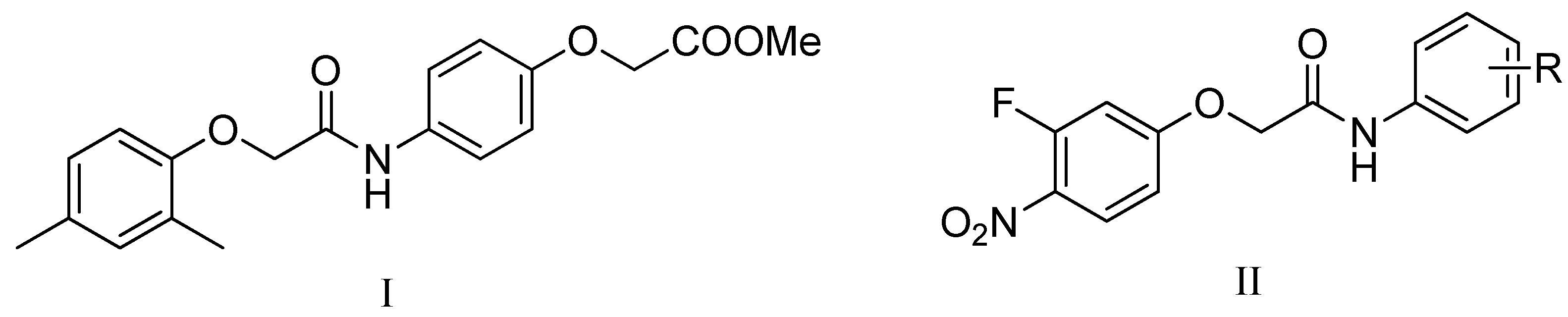
2. Results and Give-and-take
2.ane. Chemistry
To gain quick access to a range of final molecules for antitubercular activity evaluation, our initial synthetic effort was devoted to the synthesis of the pivotal intermediate, 2-(3-fluoro-iv-nitrophenoxy)-acetic acid, by employing a routine Williamson ether reaction starting from iii-fluoro-4-nitrophenol and chloroacetic acid, which tin exist further condensed with an advisable substituted aniline to afford the final compounds. Unfortunately, the simple Williamson reaction did not proceed to completion to afford the main product under the standard atmospheric condition for our specific substrates, because the fluorine cantlet ortho to the nitro group of 3-fluoro-iv-nitrophenol is prone to hydrolysis under bones conditions. Subsequently laborious chromatographic workup, 2-(iii-fluoro-4-nitrophenoxy)acetic acrid can be obtained in very low yield. The following condensation footstep with several substituted anilines by dicyclohexylcarbodiimide (DCC) strategy did not proceed to completion and column chromatography must likewise be utilized to purify the target compounds.
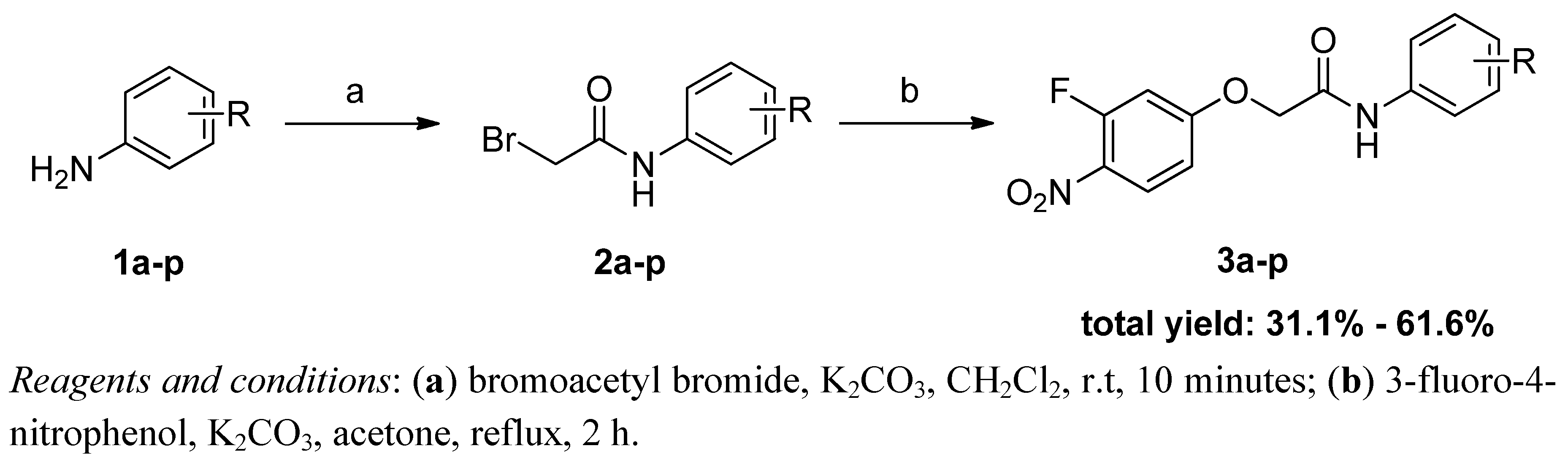
In order to brand workup more convenient, we turned to a reported reaction sequence as shown in Scheme ane [12]. In the commencement step, the intermediates 2a–p were synthesized by treating the respective aromatic amines 1a–p with bromoacetyl bromide at room temperature for 10 minutes in the presence of potassium carbonate. So, without further purification of the crude products 2a–p, the final compounds 3a–p were obtained by refluxing sure 2a–p in acetone with 3-fluoro-4-nitrophenol under bones conditions. Finally, all of the desired products 3a–p were easily obtained by crystallization from ethyl acetate.
Scheme one. Synthetic road to2-(iii-fluoro-iv-nitrophenoxy)-N-phenylacetamide derivatives 3a–p.
Scheme 1. Synthetic route to2-(3-fluoro-4-nitrophenoxy)-N-phenylacetamide derivatives 3a–p.
2.2. Antitubercular Activeness
All the final compounds were evaluated in vitro as anti-tubercular agents by a microdilution method and their MIC values were shown in Table 1.
Tabular array 1. The MIC90 and MIC values of 2-(iii-fluoro-4-nitrophenoxy)-N-phenylacetamide derivatives 3a–p against M. tuberculosis H37Rv.
| Compd. | R | MIC90 a (μg/mL) | MIC b (μg/mL) |
|---|---|---|---|
| 3a | iii-Cl | 4 | 16 |
| 3 b | 3,4-Clii | 16 | 32 |
| 3 c | four-Br,2-F | 16 | 32 |
| iii d | four-Cl,3-CF3 | 8 | 16 |
| 3 e | iii-NO2 | 16 | 64 |
| 3 f | 2,four-(OCHiii)2 | xvi | 64 |
| 3 g | 3,5-(CF3)2 | 4 | viii |
| 3 h | 4-F,3-Cl | 8 | 16 |
| 3 i | 4-Br | 16 | 32 |
| 3 j | 2-CN | 16 | 64 |
| 3 1000 | 2-CF3 | 32 | 64 |
| 3 l | 4-OCF3 | sixteen | 32 |
| three one thousand | 2-NO2 | 1 | 4 |
| iii due north | four-Br,2-Cl | 16 | 32 |
| 3 o | 4-CFthree | viii | 16 |
| 3 p | five-NOtwo,ii-Me | 64 | 64 |
| I | - | 1 | 4 |
| Isoniazide | - | 0.0625 | 0.0625 |
To annotation, in our hands the MIC90 (ane μg/mL) or MIC (4 μg/mL) value of the reported hit compound I was less potent than stated. Compound 3m with an o-nitroaniline moiety in the due east part of the 2-phenoxy-Due north-phenylacetamide scaffold, showed an identical MIC value to the hit compound I, being the all-time active ane amidst the novel 2-(3-fluoro-4-nitrophenoxy)-Due north-phenylacetamide derivatives with an MIC value of 4 μg/mL. However, compound 3e and chemical compound 3p with a nitro grouping at the meta-position showed low inhibitory effects on H37Rv bacterial strain with an MIC value of 64 μg/mL. The underlying mechanism of this difference is not articulate. The aniline bearing a trifluoromethyl group exhibited meliorate activity (compound 3d and compound 3o with an identical MIC value of sixteen μg/mL) except for ortho-trifluoromethyl (compound 3k with an MIC values of 64 μg/mL). 1 chlorine atom in the aniline part (compound 3a with an MIC value of 16 μg/mL) generated the slightly higher antitubercular activity relative to two chlorines (chemical compound 3b with an MIC value of 32 μg/mL) or 1 bromine atom substituent (compound 3i with an MIC value of 32 μg/mL). Overall all the novel 2-(3-fluoro-4-nitrophenoxy)-Due north-phenylacetamide derivatives listed in Table 1 exerted potent or moderate active against 1000. tuberculosis H37Rv, which indicates that the ii-phenoxy-N-phenylacetamide scaffold is ideal for develop novel antitubercular agents by structural modification through various side chain introduction since our SARs information suggesting reasonable tolerance of functional groups changes.
Compound 3m was further examined for its antitubercular activities against two resistant Thousand. tuberculosis strains isolated from clinical cases and the results were reported in Table ii. As shown in Table ii, compound 3m showed skilful activity against rifampicin-resistant TB with MIC of 4 μg/mL, and it also exhibited moderate action against isoniazide-resistant TB with an MIC value of 32 μg/mL.
Tabular array 2. The MIC values of chemical compound 3m confronting two resistant Yard. tuberculosis strains isolated from clinical cases a.
| Compd. | MIC b (μg/mL) | |
|---|---|---|
| Isoniazide-resistant TB 242 | Rifampicin-resistant TB 261 | |
| 3m | 32 | 4 |
| I | 16 | 4 |
| Isoniazide | 0.v | 0.125 |
| Rifampicin | ≤0.25 | >32 |
ii.iii. MTT-Based Cytotoxicity Analysis
Compounds with MIC values ranging from four μg/mL to sixteen μg/mL (compounds 3a, 3d, 3g, 3h, 3m and 3o) were chosen to deport further cytotoxicity assays. The cytotoxic effects of these derivatives against six different cell lines were determined by the MTT assay method. The IC50 values of these compounds are listed in Table 3.
Tabular array 3. The ICfifty values of some ii-(iii-fluoro-four-nitrophenoxy)-N-phenylacetamide derivativeson six tumor cell lines.
| Compd. | IC50 a (μmol/L) | ||||||
|---|---|---|---|---|---|---|---|
| A375 b | A549 c | HepG2 d | HCT116 east | SKOV-3 f | Hela 1000 | Vero h | |
| 3a | >forty.00 | >40.00 | >twoscore.00 | >40.00 | >40.00 | >40.00 | >40.00 |
| 3d | >40.00 | >forty.00 | >twoscore.00 | >40.00 | >40.00 | 33.19 ± 2.five | >forty.00 |
| 3g | 22.61 ± 0.nine | 38.nineteen ± 1.5 | 21.64 ± ane.ane | 33.84 ± 2.0 | 12.18 ± ane.3 | xx.02 ± 1.ane | >40.00 |
| 3h | >40.00 | >40.00 | >twoscore.00 | >forty.00 | >twoscore.00 | >40.00 | >twoscore.00 |
| 3m | >40.00 | >40.00 | >xl.00 | >40.00 | >forty.00 | >40.00 | >40.00 |
| 3o | 28.29 ± ane.2 | >40.00 | >40.00 | >40.00 | 28.60 ± 2.3 | 33.79 ± 1.7 | >40.00 |
Most of the compounds showed depression toxicity in this assay, and specifically the about stiff compound 3m exhibited a very good safety contour as its IC50 value was above 40 μmol/Fifty against the tested six different prison cell lines.
3. Experimental
iii.1. General
All solvents and reagents were analytical grade pure and used without farther purification. All melting points were determined on a SGW X-four Micro Melting Point apparatus and are uncorrected. 1H-NMR and thirteenC-NMR spectra were recorded on a Bruker Avance (Varian Unity Inova) 400 MHz spectrometer using TMS as internal reference chemic shift in δ, ppm. High-resolution mass spectrometry was carried out on a Waters Q-TOF Premier mass spectrometer.
3.2. Synthesis of Compounds 3a–p
Bromoacetyl bromide (1.18 eq.) was added dropwise to a mixture of the appropriate aniline 1a–p (3.00 mmol, 1.0 eq.) and K2CO3 (1.xviii eq.) in CH2Clii (10 mL) over x min. After cooling to room temperature, water (10 mL) was slowly added. The aqueous solution was extracted twice with CH2Cl2 (10 mL), the organic stage was dried over NaiiSo4, filtered and the solvent was evaporated to furnish the crude product 2a–p, which used in the side by side step without further purification (spectroscopic data were in understanding with published data).
A mixture of the advisable intermediate 2a–p (one.0 eq.), iii-fluoro-iv-nitrophenol (1.0 eq.), Chiliad2COthree (one.2 eq.) and KI (0.i eq.) in acetone (20 mL) was stirred at room temperature for 5 min and then refluxed for ii h. The solvent was evaporated to requite a residue which was suspended in HiiO (xx mL) and extracted twice with EtOAc (20 mL). After drying over anhydrous Na2And then4, the organic layer was filtered and the solvent evaporated to dryness. The desired pure products 3a–p were finally obtained past crystallization from EtOAc.
Due north-(3-Chlorophenyl)-2-(3-fluoro-4-nitrophenoxy)acetamide (3a): Stake yellowish solid, full yield for the two steps: 48.7%; m.p. 150–151 °C; aneH-NMR (DMSO-d6 ): δ 10.39 (south, 1H), 8.19 (t, J = 1.6 Hz, 1H), seven.82 (s, 1H), vii.l (d, J = 8.iv Hz, 1H), seven.37 (t, J = 8 Hz, 1H), 7.27 (d, J = 6.8 Hz, 1H), 7.xvi (d, J = 7.vi Hz, 1H), seven.05 (d, J = ix.2 Hz, 1H), 4.95 (south, 2H); 13C-NMR (DMSO-d6 ): δ 165.98, 164.05, 158.05, 155.45, 139.95, 133.36, 130.80, 128.29, 123.79, 119.24, 111.94, 104.66, 67.68; HRMS (TOF) 1000/z calcd. for C14H10ClFN2O4 [G+Na+]: 324.0313, constitute: 347.0221.
Northward-(3,4-Dichlorophenyl)-two-(3-fluoro-iv-nitrophenoxy)acetamide (3b): Yellow solid, total yield for the 2 steps: 61.6%; m.p. 175–176 °C; 1H-NMR (DMSO-d 6 ): δ 10.49 (s, 1H), 8.19 (t, J = 9.2 Hz, 1H), 7.80 (d, J = 13.half dozen Hz, 1H), vii.62-7.53 (m, 2H), 7.27 (dd, J = 13.half-dozen Hz, 2.4 Hz, 1H), vii.05(dd, J = 8.8 Hz, ii Hz, 1H), iv.95 (due south, 2H); 13C-NMR (DMSO-d6 ): δ 166.08, 163.93, 157.99, 155.39, 138.53, 131.25, 130.96, 128.24, 125.51, 121.02, 119.84, 104.73, 104.48, 67.61; HRMS (TOF) m/z calcd. for CxivH9Cl2FN2O4 [Grand+Na+]: 382.9792, institute: 382.9813.
N-(4-Bromo-ii-fluorophenyl)-2-(iii-fluoro-4-nitrophenoxy)acetamide (3c): Pale yellow solid, total yield for the 2 steps: 51.5%; m.p. 160–162 °C; 1H-NMR (DMSO-dvi ): δ 10.xvi (s, 1H), viii.13 (t, J = 8.8 Hz, 1H), 7.83 (t, J = eight Hz, 1H), 7.64 (d, J = 10 Hz, 1H), 7.41 (d, J = 8 Hz, 1H), 7.24 (d, J = 13.2 Hz, 1H), 7.03 (d, J = viii.8 Hz, 1H), v.01 (s, 2H); thirteenC-NMR (DMSO-d6 ): δ 166.xviii, 164.11, 158.05, 155.34, 152.74, 128.27, 127.86, 125.93, 125.22, 119.29, 116.lxx, 111.91, 104.59, 67.61; HRMS (TOF) m/z calcd. for C14HnineBrF2N2O4 [G+H+]: 386.9792, found: 387.1709.
N-(four-Chloro-3-(trifluoromethyl)phenyl)-2-(3-fluoro-4-nitrophenoxy)acetamide (3d): Pale yellow solid, full yield for the two steps: 58.9%; g.p. 163–164 °C; 1H-NMR (DMSO-d6 ): δ 10.58 (s, 1H), 8.21–8.16 (m, 2H), 7.90 (dd, J = eight.8 Hz, 2 Hz, 1H), 7.70 (d, J = viii.8 Hz, 1H), 7.27 (dd, J = xiii.6 Hz, 2.4 Hz, 1H), 7.06 (dd, J = 9.2 Hz, two Hz, 1H), 4.97 (s, 2H); 13C-NMR (DMSO-d6 ): δ 166.36, 163.95, 158.05, 155.45, 137.99, 132.46, 128.26, 127.02, 124.72, 124.27, 121.55, 118.53, 111.98, 104.66, 67.65; HRMS (TOF) m/z calcd. for CxvHixClF4N2O4 [One thousand+Na+]: 415.0085, found: 415.0099.
two-(3-Fluoro-four-nitrophenoxy)-N-(3-nitrophenyl)acetamide (3e): Yellow solid, total yield for two steps: 34.4%; m.p. 199–201 °C; 1H-NMR (DMSO-dhalf-dozen ): δ 10.62 (s, 1H), viii.63 (s, 1H), eight.19 (t, J = eight.viii Hz, 1H), 8.00–7.95 (m, 2H), vii.65 (t, J = eight.4 Hz, 1H), vii.28 (dd, J = xiii.6 Hz, 2.4 Hz,1H), 7.08 (dd, J = 9.2 Hz, 2 Hz, 1H), four.99 (southward, 2H); 13C-NMR (DMSO-dvi ): δ 166.42, 163.99, 158.05, 155.46, 148.xviii, 139.64, 130.68, 128.28, 125.86, 118.60, 113.99, 111.97, 104.68, 67.66; HRMS (TOF) k/z calcd. for CfourteenHxFN3Ohalf dozen [K+Na+]: 358.0451, establish: 358.0467.
N-(two,4-Dimethoxyphenyl)-two-(3-fluoro-4-nitrophenoxy)acetamide (3f): Yellow solid, full yield for the two steps: 38.8%; thou.p. 174–175 °C; oneH-NMR (DMSO-d6 ): δ 9.31 (southward, 1H), 8.19 (t, J = 8.viii Hz, 1H), 7.71 (d, J = 8.8 Hz, 1H), seven.25 (dd, J = 9.6 Hz, 2.4 Hz, 1H), seven.04 (dd, J = 9.2 Hz, 1.half dozen Hz, 1H), half-dozen.64 (d, J = 2.4 Hz, 1H), 6.50 (dd, J = 8.8 Hz, two.8 Hz, 1H), 4.94 (southward, 2H), 3.83 (s, 3H), 3.75 (s, 3H); 13C-NMR (DMSO-d6 ): δ 165.25, 163.99, 158.08, 157.47, 155.48, 151.76, 130.68, 128.29, 123.77, 119.58, 112.00, 104.50, 99.11, 67.89, 56.02, 55.56; HRMS (TOF) m/z calcd. for C16HxvFN2Ovi [M+]: 350.0914, plant: 350.0787.
N-(3,5-bis(Trifluoromethyl)phenyl)-2-(3-fluoro-4-nitrophenoxy)acetamide (3g): Pale yellowish solid, total yield for two steps: 32.8%; one thousand.p. 168–169 °C; 1H-NMR (DMSO-dhalf dozen ): δ x.82 (s, 1H), eight.32 (s, 2H), 8.xx (t, J = ix.2 Hz, 1H), 7.84 (south, 1H), vii.31 (dd, J = 13.6 Hz, 2.4 Hz, 1H), 7.09 (dd, J = 9.two Hz, two.four Hz, 1H), 5.01 (s, 2H); xiiiC-NMR (DMSO-d6 ): δ 167.05, 164.03, 157.80, 156.07, 140.60, 131.25, 128.45, 126.31, 124.50, 122.70, 120.89, 119,83, 117.09, 112.23, 104.96, 104.80, 67.83; HRMS (TOF) k/z calcd. for C16H9FviiN2O4 [G+Na+]: 449.0348, establish: 449.0328.
N-(iii-Chloro-iv-fluorophenyl)-ii-(iii-fluoro-4-nitrophenoxy)acetamide (3h): Xanthous solid, total yield for two steps: 56.8%; m.p. 166–167 °C; 1H-NMR (DMSO-dsix ): δ 10.xl (s, 1H), viii.20 (t, J = 8.8 Hz, 1H), seven.93 (dd, J = half dozen.viii Hz, 2.iv Hz, 1H), 7.55–7.51 (g, 1H), 7.41 (t, J = 9.ii Hz, 1H), 7.27 (dd, J = 13.6 Hz, two.4 Hz, 1H), 7.06 (dd, J = 9.2 Hz, ii Hz, 1H), 4.94 (s, 2H); 13C-NMR (DMSO-dsix ): δ 166.08, 164.17, 157.80, 156.06, 154.68, 153.07, 135.xc, 130.96, 128.45, 121.60, 120.47, 119.64, 117.45, 112.16, 104.86, 67.88; HRMS (TOF) m/z calcd. for C14HixClF2Due north2Oiv [M+Na+]: 365.0117, found: 365.0143.
Northward-(4-Bromophenyl)-two-(three-fluoro-iv-nitrophenoxy)acetamide (3i): Stake yellow solid, total yield: 42.two%; m.p. 166–167 °C; 1H-NMR (DMSO-dhalf dozen ): δ 10.33 (s, 1H), 8.19 (t, J = viii.8 Hz, 1H), 7.59 (d, J = 8.8 Hz, 1H), 7.52 (d, J = eight.eight Hz, 2H), seven.26 (dd, J = xiii.2 Hz, i.vi Hz, 1H), 7.05 (dd, J = 9.2 Hz, 2.4 Hz, 1H), four.94 (due south, 2H); thirteenC-NMR (DMSO-dsix ): δ 165.91, 164.28, 157.81, 156.07, 138.08, 132.08, 130.93, 128.45, 122.00, 115.92, 112.12, 104.84, 67.97; HRMS (TOF) g/z calcd. for C14H10BrFN2Oiv [Thousand+Na+]: 392.9685, found: 392.9710.
N-(2-Cyanophenyl)-2-(3-fluoro-four-nitrophenoxy)acetamide (3j): White solid, full yield for the two steps: 31.ane%; m.p. 172–174 °C; 1H-NMR (DMSO-d6 ): δ 10.45 (s, 1H), 8.20 (t, J = nine.two Hz, 1H), 7.86 (d, J = vii.6 Hz, 1H), 7.52 (d, J = 8.8 Hz, 2H), 7.73 (t, J = 8 Hz, 1H), seven.65 (d, J = 8 Hz, 1H), 7.41 (t, J = half-dozen.8 Hz, 1H), 7.27 (dd, J = 13.half-dozen Hz, 2 Hz, 1H), 7.07 (d, J = 9.2 Hz, 1H), 5.03 (due south, 2H); 13C-NMR (DMSO-d6 ): δ 166.63, 164.02, 157.81, 156.08, 139.70, 134.41, 133.74, 131.04, 128.43, 126.73, 126.fifteen, 117.fourteen, 112.36, 108.13, 104.87, 67.88; HRMS (TOF) m/z calcd. for C15H10FNiiiOiv [Grand+Na+]: 338.0553, constitute: 338.0544.
two-(iii-Fluoro-four-nitrophenoxy)-Due north-(2-(trifluoromethyl)phenyl)acetamide (3k): colorless crystal, full yield for the 2 steps: 39.7%; m.p. 146–147 °C; 1H-NMR (DMSO-d6 ): δ 9.94 (s, 1H), viii.xx (t, J = 9.2 Hz, 1H), 7.78 (d, J = half-dozen.viii Hz, 1H), 7.72 (t, J = 8 Hz, 1H), vii.57 (d, J = 8 Hz, 1H), 7.51 (t, J =vii.half dozen Hz, 1H), vii.22 (dd, J = thirteen.ii Hz, 2.4 Hz, 1H), seven.04 (dd, J = 9.ii Hz, ii Hz, 1H), 4.98 (s, 2H); 13C-NMR (DMSO-d6 ): δ 166.99, 163.99, 157.79, 156.06, 134.89, 133.66, 131.00, 128.44, 127.67, 126.89, 124.86, 123.05, 112.31, 104.71, 67.85; HRMS (TOF) m/z calcd. for CxvHxF4N2O4 [Thou+Na+]: 381.0474, establish: 381.0459.
2-(iii-Fluoro-4-nitrophenoxy)-N-(4-(trifluoromethoxy)phenyl)acetamide (3l): Grey white solid, total yield for the two steps: twoscore.0%; g.p. 146–148 °C; oneH-NMR (DMSO-d6 ): δ 10.40 (s, 1H), 8.nineteen (t, J = 9.ii Hz, 1H), 7.74–7.71 (grand, 2H), 7.35 (d, J = 8.4 Hz, 2H), 7.26 (dd, J = 13.half dozen Hz, two.8 Hz, 1H), vii.05 (dd, J = ix.ii Hz, 2 Hz, 1H), 4.95 (due south, 2H); 13C-NMR (DMSO-d6 ): δ 165.99, 164.28, 155.65, 144.42, 137.90, 130.96, 128.45, 121.85, 119.31, 112.14, 104.85, 67.96; HRMS (TOF) m/z calcd. for C15H10FfourNorthiiO5 [M+K+]: 397.0214, found: 397.0431.
2-(3-Fluoro-iv-nitrophenoxy)-N-(2-nitrophenyl)acetamide (3m): Xanthous solid, total yield for the two steps: 39.2%; g.p. 201–202 °C; aneH-NMR (DMSO-d6 ): δ x.77 (south, 1H), viii.22 (t, J = nine.2 Hz, 1H), 8.07 (dd, J = viii Hz, one.2 Hz, 1H), 7.95 (d, J = viii Hz, 1H), 7.79–7.75 (m, 1H), 7.44-seven.39 (one thousand, 1H), 7.27 (dd, J = 13.6 Hz, two.8 Hz, 1H), 7.08 (dd, J = 9.2 Hz, ii.4 Hz, 1H), 5.00 (s, 2H); 13C-NMR (DMSO-d6 ): δ 166.47, 163.60, 158.25, 155.65, 141.48, 135.16, 131.25, 128.51, 125.84, 125.09, 122.48, 104.85, 67.88; HRMS (TOF) m/z calcd. for CfourteenH10FN3Osix [M+Na+]: 358.0451, plant: 358.0452.
N-(4-Bromo-two-chlorophenyl)-2-(3-fluoro-iv-nitrophenoxy)acetamide (3n): White solid, total yield for two steps: 54.1%; 1000.p. 173–175 °C; 1H-NMR (DMSO-d6 ): δ ix.90 (s, 1H), 8.twenty (t, J = 9.2 Hz, 1H), 7.83 (d, J = 2 Hz, 1H), 7.73 (d, J = viii.eight Hz, 1H), vii.58 (dd, J = 8.four Hz, 2 Hz, 1H), 7.27 (dd, J = nine.2 Hz, 2.viii Hz, 1H), 7.06 (dd, J = 9.2 Hz, ii Hz, 1H), 5.02 (s, 2H); xiiiC-NMR (DMSO-d6 ): δ 166.37, 164.04, 158.24, 155.64, 134.12, 132.18, 131.06, 128.39, 127.76, 118.25, 112.23, 104.89, 67.95; HRMS (TOF) m/z calcd. for C14H9BrClFN2Ofour [K+Na+]: 426.9295, found:426.9032.
2-(iii-Fluoro-4-nitrophenoxy)-N-(four-(trifluoromethyl)phenyl)acetamide (3o): Pale yellow solid, total yield for the two steps: 36.one%; m.p. 173–175 °C; 1H-NMR (DMSO-d6 ): δ 10.57 (s, 1H), 8.19 (t, J = 9.6 Hz, 1H), seven.84 (d, J = 8.8 Hz, 2H), vii.72 (d, J = eight.4 Hz, 2H), 7.28 (dd, J = 13.6 Hz, ii.4 Hz, 1H), 7.06 (dd, J = ix.ii Hz, two Hz, 1H), four.99 (s, 2H); 13C-NMR (DMSO-d6 ): δ 166.42, 164.27, 158.25, 155.65, 142.31, 130.96, 128.45, 126.54, 126.ten, 124.28, 123.40, 119.96, 112.13, 104.85, 67.95; HRMS (TOF) thou/z calcd. for CxvHtenF4NiiOiv [M+Na+]: 381.0474, found: 381.0473.
2-(three-Fluoro-iv-nitrophenoxy)-N-(2-methyl-5-nitrophenyl)acetamide (3p): Pale yellowish solid, total yield for 2 steps: 36.9%; chiliad.p. 211–213 °C; 1H-NMR (DMSO-dhalf dozen ): δ 9.ninety (s, 1H), 8.44 (d, J = 2.4 Hz, 1H), 8.21 (t, J = 9.2 Hz, 1H), 7.99 (dd, J = 8.four Hz, 2.4 Hz, 1H), 7.54 (d, J = eight.8 Hz, 1H), 7.29 (dd, J = 13.6 Hz, 2.4 Hz, 1H), seven.08 (dd, J = ix.2 Hz, 2 Hz, 1H), 5.04 (s, 2H), 2.36 (south, 3H); thirteenC-NMR (DMSO-d6 ): δ 166.57, 164.24, 158.27, 155.67, 146.eighteen, 140.07, 136.88, 131.96, 130.99, 128.48, 120.36, 119.26, 112.19, 104.86, 67.ninety; HRMS (TOF) 1000/z calcd. for C15H12FN3Ohalf-dozen [K+Na+]: 372.0608, found: 372.0599.
three.3. Antitubercular Activity
Middlebrook7H9 medium dry out powder and diet supplements (OADC) were bought from the Becton Dickinson (BD) Company (Franklin Lakes, NJ, United states of america). The M. tuberculosis H37Rv (ATCC 27294) were provided by national strains preservation center. Clinical isolated single-drug resistant Yard. tuberculosis H37Rv were provided past the Shanghai Pulmonary Hospital.
Organisms for testing were initially grown to turbidity in 7H9 broth for 14 days. The cultures were vortexed for 20 to thirty seconds before apply and so left for ten to 30 minutes to allow for settling of heavy particles. The interruption of organisms was and then matched to the optical density of a ane.0 McFarland standard. Appropriate dilutions were added to the wells of the microtiter plates containing the dissimilar drug dilutions past using a disposable inoculator. The tested compounds were added to the medium equally DMSO solutions. The following concentrations were used: 64, 32, sixteen, 8, 4, two, 1, 0.five, 0.25, 0.125, 0.0625, 0.03125 μg/mL. The plates were sealed in plastic numberless and incubated at 37 °C in room air in a moisturized incubator. Isoniazide (Sigma-Aldrich, St. Louis, MO, Us) was used every bit positive control in each experiment, and each evaluation was performed in triplicate. The plastic numberless were essential to prevent evaporation in the wells and were also useful for biologic containment. All microdilution plates were read after 11 days by visual investigation with an indirect calorie-free source.
3.4. MTT-Based Cytotoxicity Assay
Briefly, cells (2,500/well) were seeded in 96-well plates and cultured for 24 h, followed by handling with the target compounds for some other 48 h. MTT (v μg/mL, 20 μL) was added per well and incubated for another 2.5 h at 37 °C. And so the supernatant fluid was removed and 150 μL/well DMSO was added for fifteen–20 min. The absorbance (OD) of each well was measured at 570 nm, using a SpectraMAX M5 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, U.s.). The IC50 Values are ways ± SEM of three independent experiments.
4. Conclusions
Sixteen two-(iii-fluoro-4-nitrophenoxy)-Northward-phenylacetamide derivatives were synthesized and their antimycobacterial activities against K. tuberculosis H37Rv evaluated past a microdilution method. The newly synthesized derivatives take high to moderate potency against One thousand. tuberculosis H37Rv, which indicates that the readily available 2-phenoxy-N-phenylacetamide scaffold is platonic for developing novel affordable antitubercular agents by structural modification. Six compounds exhibited potent antitubercular activity, with MIC values ranging from iv to 16 μg/mL, and ii-(3-fluoro-iv-nitrophenoxy)-Due north-(2-nitrophenyl)acetamide (compound 3m) was proved to be the about stiff inhibitor with an identical MIC value of 4 μg/mL for Chiliad. tuberculosis H37Rv and rifampin-resistant M. tuberculosis 261 strain isolated from clinical cases. It also has a good safety profile in 6 dissimilar cell lines by the MTT assay, indicating compound 3m exist a expert lead for subsequent optimization to get better antitubercular agents.
tillettwoutiornow39.blogspot.com
Source: https://www.mdpi.com/1420-3049/17/2/2248/htm
0 Response to "in order to synthesize n-phenyl acetamide what should you combine aniline with?"
Postar um comentário